Overview
Research Focus
Select Publications
Facilities
Projects
Research Staff
Overview
Energy has been called the ultimate resource for two reasons. Primarily, without energy resources on the planet are unobtainable. Secondly, unlike water or carbon, energy cannot itself be re-cycled. Industrialized countries have come to depend heavily on large amounts of energy to support life and it is expected that it will always be there at affordable prices. The global energy use is expected to increase with 60 % during the next 30 years. At the same time, there is an urgent need to decrease greenhouse gas emissions, which are mainly responsible for the actual climate changes. Limited access, increasing price and geographical uncertainty in the access regions to natural resources such as coal, oil, gas, and uranium make the development and use of sustainable energy sources a global necessity.
Research Focus
In this regard, the Energy group is exploring to produce low cost alternate renewable eco-friendly biofuels from unused materials such as non edible oil seeds and waste cooking oil and the team is exploring potential alternate techniques to the current biodiesel production methods for significant increase in the production capacity and product quality to reduce cost and the reduction in the reaction time, thus contributing to biodiesel commercialization.
The research team has explored various techniques namely ultrasonic and microwave processes for the production of biodiesel with different feedstock as alternatives to the conventional method. Produced biodiesels have been confirmed by Fourier Transform Infra Red spectroscopy (FTIR) and Gas Chromatography (GC) techniques. Standardization of biodiesels has been done as per ASTM G6751 for confident usage of produced biodiesels in existing engines. Performance, emission and combustion characteristics of different methyl esters also called biodiesels and their diesel blends in a C.I. engine are also examined.
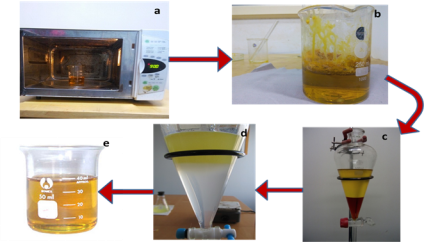
Microwave assisted Transesterification process


4 stroke single cylinder Engine Exhaust gas analyzer
The existing fuel infrastructure must be able to accommodate as many of these new fuels as possible, to allow the greatest flexibility in adoption and utilization of renewable, eco-friendly petroleum alternatives. If an alternate fuel industry for fossil fuel develops, there is an urgent need for innovative research to prevent undesirable consequences in engines, generators, pump sets and other existing fuel infrastructure and to make the most of the potential benefits of alternative fuels. In this context, investigation on the compatibility of various biodiesels and their different blends with commercial diesel on a few industrial metals such as aluminium, copper, brass and mild steel is focussed mainly. Emulsion inversion point and wettability are determined to understand the impact of biofuels on the selected metals. Common methods adopted for measurement of corrosion include mass loss through static immersion tests and Linear Polarization Resistance measurements. The effects of flow and dissolved oxygen on the metal corrosion are studied using rotating cage.

Rotating cage is a promising and reliable method to simulate pipeline flow under laboratory conditions to evaluate the corrosion rate of eight coupons simultaneously. Figure to the right shows the rotating cage used in the laboratory, which has been fabricated as per ASTM G184. Experiments are conducted for a period of 100h at the rotation speed of 500 rpm. The rate of corrosion of metals is calculated from the difference in mass of the coupons while the deterioration of plastics and elastomers is also analyzed. Corrosion kinetics and the thermodynamic functions are studied for the metal dissolution from temperature study. The change in surface of the materials due to corrosion reaction is analyzed by optical microscopy, scanning electron microscopy and the localized corrosion by laser profilometry. Elemental analyses of tested coupons in biofuels are characterized by Energy Dispersive X Ray Spectroscopy (EDS) and X Ray Diffraction (XRD).
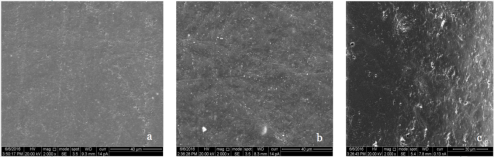
SEM Micrographs of polymer coupons
- Untreated surface b. Exposed to B100 (100%biodiesel) at 25 oC
- Exposed to B100 at 75 oC
Biofuel degrades through auto oxidation, moisture absorption, attack by microorganisms, etc. during storage or use. The currently used indicators of corrosiveness of biodiesel – copper strip corrosion and TAN value that are prescribed by different standards are not effective enough. There is a serious lack of scientific data on corrosion of automotive metals and alloys in biodiesel based on which confident decisions could be made. More data are necessary to substantiate confident utilisation of alternate fuels in automotive industry. With biodiesel production and consumption continuing to be a major component of the motor-fuel pool, there is a profound need for materials and equipment that are capable of handling its production, transportation, handling and storage. Oxidative stability and fuel degradation of commercially available biofuel on elastomeric materials, polymers and as such engine parts are also being investigated.
Further, seed cakes/meals obtained as co-products during production of oil from non-edible seeds such as neem, jatropha and pongamia contain high amounts of proteins that may have unique properties. These meals contain up to 50% proteins that could have antimicrobial, antioxidant and anticancer properties and are potentially useful in food, medical and cosmetic applications. Hence, proteins have been extracted from different types of non-edible oil seed meals and films are being developed from the extracted proteins. The films formed are used to evaluate the antibacterial, antimicrobial and anticancer properties using standard techniques which can be used for product development. Also, extracted non edible oils can be used for developing products like face pack, face creams, ointments and other cosmetic applications. This activity is being jointly coordinated with the biocomposites group.

Seed Meal and Protein film
Biodiesel production unit
A batch scale biodiesel production unit was established on the 10th of August 2017, marking the 125th anniversary of Rudolf Diesel’s peanut oil experiments on his diesel engine in 1893. The unit would initially produce about 200 litres of biodiesel on a daily basis (with scope for increasing the production capacity) apart from producing other value added products such as floor disinfectants and soap. The objective of this installation is to promote the use of a clean and renewable fuel such as biodiesel in and around the Tataguni area in southern Bengaluru by not only utilizing the biodiesel for utilization on campus such as for the buses and generators but also for sale of this fuel to other companies in the region. The aim is to develop a green campus. The unit’s functioning is in line with the Indian Government’s ‘Make in India’ and ‘Swachh Bharat Abhiyaan’ initiatives.
Redox Flow Battery
The mounting concerns over the impending dangers attributable to the fast depletion of traditional sources of energy such as fossil fuels command sustainable solutions for energy insecurity and greenhouse gas emissions. Electrochemical storage devices particularly redox flow batteries (RFBs) come across as promising choices for grid-scale storage system. There are different types of RFB systems such as iron-chromium, bromine-polysulfide, iron-vanadium, all-vanadium, vanadium-bromine, vanadium-oxygen, zinc-bromine, etc. that have been the topic of intense investigations. In spite of being advantageous, these RFBs face challenges in terms of cost, availability and eco-friendliness. The all-iron redox flow batteries present an attractive solution because of the use of inexpensive materials, abundantly available iron and non-toxic nature of the system.
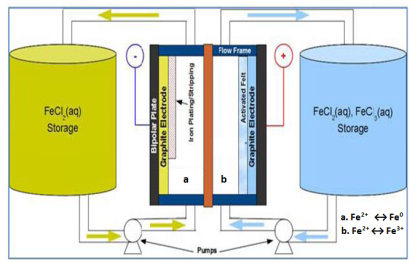
The design of all-iron redox flow battery plays a pivotal role in deciding the total amount of energy that can be stored in the system. The components of all-iron redox flow battery and electrolyte solutions in the external storage tanks greatly influence the performance and the costs of all-iron redox flow battery. The ratio of anolyte and catholyte solutions is a function of state of charge (SOC). The cost of all-iron redox flow battery is directly proportional to the cost of metal salts used as the electrolyte. Chemicals such as ammonium chloride (NH4Cl) and boric acid (H3BO3) may be added to the electrolyte so as to bring down the resistivity of the electrolyte and to inhibit the hydrogen evolution respectively. Pumps help in the movement of electrolytes in and out of the cell stack. Bipolar plates provide structural support and act as a conductor. In the stacked configuration, they work as positive electrode for one cell and negative for adjacent cell. The electrodes present next to the bipolar plates allow the electrochemical reactions to occur on surface. The total energy output of all-iron redox flow battery will depend on the amount of metal deposited on the electrode surface. They enable the electrons to move in or out of the electrolyte. The electrode used in all-iron battery is usually graphite based. Other important parts of all-iron redox flow battery are membrane separator, flow frame and control system whose functions are to acting as a boundary between the electrolytes so that they do not mix, imparting a structure for movement of electrolyte in and out of the stack and providing a computerized controlling of flow rates, charge as well as discharge currents respectively. Typically the membrane separator employed is an ion exchange membrane such as nafion membrane.
The research aims to develop and demonstrate all – iron redox flow battery systems, utilizing earth-abundant, iron salt and water as its electrolyte, and carbon based electrodes as battery components. Towards achieving this goal, focus is on screening and optimization of cell and stack components, including electrolytes, electrodes, current-collecting bi-polar plates, and proton membrane or separator.
Select Publications
Comparison on the Corrosion rates of Copper, Zinc and Brass in Pongamia and Jatropha Biodiesels. Meenakshi HN., Anisha A., Shyamala R., Saratha R. Indian Journal of Chemical Technology (2017)24:417-423. IF:0.568
Deterioration of automotive polymeric materials in exposed to Pongamia pinnataMeenakshi H.N, Anish Prasad Sah and Rajesh Sah. Asian Journal of Chemistry (2017) 29(7): 1471 –1476. IF:0.15
Facilities
Electrochemical work station (CH Instruments, USA), Rotating cage, Pulse plating rectifiers, Water bath, Ultrasonic bath, Muffle Furnace, Vacuum oven, Water distillation unit, Hot air oven, Deep freezer, Fischer Centrifugation unit.
Projects
Compatibility of fueling infrastructure materials with methanol and gasoline blends- DST
Development of low-cost Fe based flow batteries for grid level energy storage- DST
Production of biofuels and Compatibility of fuelling infrastructure materials with different biofuels and blends with fossil fuels- SSPS
Research Staff

Muralidhara completed his Master’s and Ph.D from Kuvempu University, Karnataka and joined IISc, Bengaluru as a Post-doctoral fellow in the year 2008. Subsequently he got into the academic domain and worked as Head-R&D Chemistry at KSIT and Center for Emerging Technologies, Jain University, Bengaluru. Muralidhara’s main research domain includes electrochemistry and water purification. Research on Electrochemistry involves the development of low-cost Iron based flow batteries for renewable storage and electrodeposition on metals/alloys or 3D plastics for aerospace, automobile, medical and engineering applications. Research on water purification includes design and characterization of hierarchical nanostructures for removal of heavy metal ions and dyes from contaminated water. He has already completed projects funded by VTU and DOS and currently has ongoing projects from DST.
Muralidhara has authored about thirty five research articles and one book. His publications have so far been cited more than 700 times and has a h-index of 8. He has supervised two Ph.Ds, and more than thirty students at the Master’s level. Apart from basic and applied research he is also associated with projects having societal relevance and industrial/consultancy projects in the area of environment and electrochemistry. Muralidhara is the recipient of “MRSI Medal” by Materials Research Society of India. He is a life member for several bodies and reviewer for reputed publishers like Elsevier, Springer and Taylor and Francis. Also he is the recipient of best oral award in conferences, top cited articles award and best reviewer award from Elsevier. He has delivered many invited/plenary lectures and is currently working as an Associate Professor.
Email Id: muralidhara.hb@ciirc.jyothyit.ac.in

Meenakshi holds a doctorate in Chemistry from Avinashlingam University, Coimbatore in 2013 for the work she conducted in the area of biodiesel compatibility on industrial metals. Being a chemist, she has been motivated to execute research on corrosion of metals and their inhibition, biofuels and compatibility of biofuels with automotive materials during her masters and doctorate. She worked as a Research Member in DST sponsored Research project entitled “Corrosion behavior of few industrial metals in selected biodiesels”. She has been a recipient of DST-CURIE fellowship from Department of Science and Technology – Consolidation of University Research for Innovation and Excellence. Also, she has been awarded the prestigious Commonwealth Professional Fellowship from UK Department for International Development to enhance knowledge and skills through specialized training at Robert Gordon University, Aberdeen besides the proficiency prize at the post graduate level in 2004 at Avinashilingam University, Coimbatore. She has presented several papers at various national and international conferences and has published about seventeen papers in various national and international journals.
She worked as a Research Scientist at JU. She has built a conducive research and teaching environment by guiding graduate and post graduate students. In addition to this, she has been successful in a very short span to rope in a good amount of grants from different sources like VGST, KSCST, DST etc. to support her research and development activities and currently working as an Assistant Professor.
Email Id: meenakshi.hn@ciirc.jyothyit.ac.in

Avinash Narayanaswamy completed his Bachelors in Chemical Engineering from Rashtriya Vidyalaya College of Engineering (RVCE), Bangalore in 2004 and followed it up with two Masters Degrees in the fields of Sustainable Energy Technology and Environmental and Energy Management from the University of Twente, the Netherlands in 2009 and 2015 respectively. His passions are mainly related to clean fuels and environmental protection and thus his interest in being part of eco-friendly trips such as the ones on biodiesel. His first trip on 100% biodiesel was across mainland Europe in 2010 covering ten European countries and followed it up with another trip across five south Indian states also on 100% biodiesel in 2011. Avinash has been featured regularly on both print and digital media both in Netherlands and India. Avinash has also successfully completed two online courses: Climate Change Science and Negotiations from the University of Columbia- New York and Planetary Boundaries and Human Opportunities from the Stockholm Resilience Centre- Sweden. His other passions include traveling, wildlife and nature photography, hiking, working for the underprivileged and physically and mentally challenged children, animals in distress and promoting vegetarianism. With close to 8 years of prior work experience in the field of chemical engineering and bioenergy, Avinash is currently working as an Assistant Professor.
Email Id: avinash.n@ciirc.jyothyit.ac.in

Chandrashekar holds a Bachelor’s degree in Chemical Engineering along with two PG Diplomas in the domains of Environment pollution control and Industrial fire safety and is working as a Research Engineer. He has nearly ten years of industrial experience and during these years he has worked with Pharma, Effluent Treatment (CETP), and Material testing companies. The expertise gained over these various domains is being utilized in furthering research in Environmental related projects such as Bio fuels and energy recovery from waste.
Email Id: chandrashekhar.r@ciirc.jyothyit.ac.in

Anarghya is a Research Fellow having completed her Master’s in Chemistry from Mangalore University. Her area of research interest includes pulse plating and energy storage devices.
Email Id: anarghya.d@ciirc.jyothyit.ac.in
